Are you looking for 'homework the gas law equations answers'? You can find all of the material on this webpage.
Table of contents
- Homework the gas law equations answers in 2021
- Gas laws worksheet pdf
- Gas law equation calculator
- Ideal gas constant
- Gas laws worksheet 1 answer key
- Chemistry gas laws worksheet answer key with work
- Combined gas law worksheet answers pdf
- Gas laws questions and answers pdf
Homework the gas law equations answers in 2021
 This picture representes homework the gas law equations answers.
This picture representes homework the gas law equations answers.
Gas laws worksheet pdf
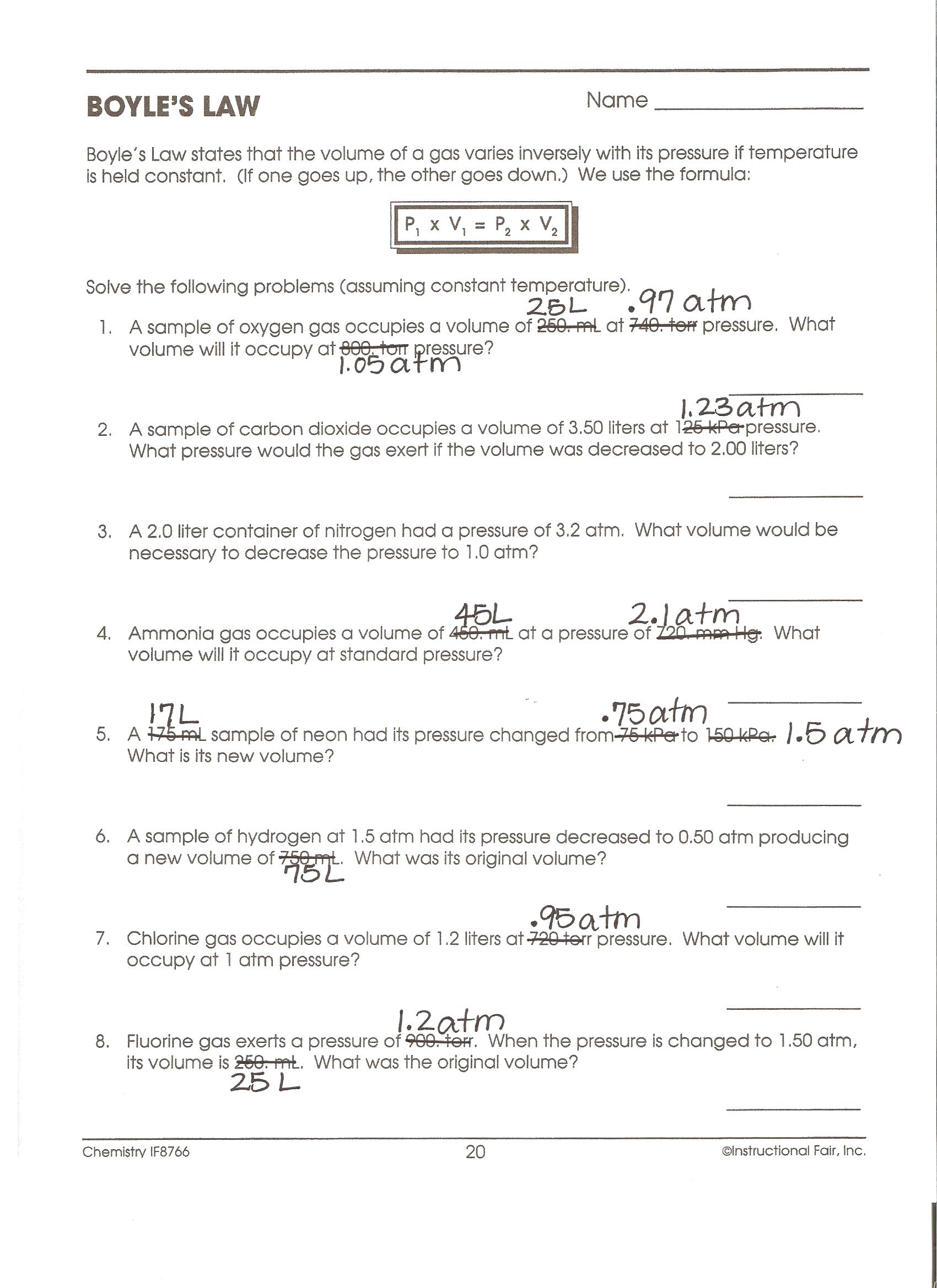 This image shows Gas laws worksheet pdf.
This image shows Gas laws worksheet pdf.
Gas law equation calculator
 This image representes Gas law equation calculator.
This image representes Gas law equation calculator.
Ideal gas constant
 This image representes Ideal gas constant.
This image representes Ideal gas constant.
Gas laws worksheet 1 answer key
 This image representes Gas laws worksheet 1 answer key.
This image representes Gas laws worksheet 1 answer key.
Chemistry gas laws worksheet answer key with work
 This picture representes Chemistry gas laws worksheet answer key with work.
This picture representes Chemistry gas laws worksheet answer key with work.
Combined gas law worksheet answers pdf
 This image shows Combined gas law worksheet answers pdf.
This image shows Combined gas law worksheet answers pdf.
Gas laws questions and answers pdf
 This image demonstrates Gas laws questions and answers pdf.
This image demonstrates Gas laws questions and answers pdf.
How are the laws of gasses related to each other?
Under standard conditions, all gasses exhibit similar behaviour. The variations in their behaviours arise when the physical parameters associated with the gas (such as temperature, pressure, and volume) are altered. The gas laws basically describe the behaviour of gases and have been named after the scientists who discovered them.
Which is unit need to be converted to gas law equations?
Which of the following do the units need to be converted to be used in gas law equations Q. A gas has a volume of 3L at 200 kPa. What will its volume be if the pressure is changed to 500 kPa? Q. A gas has a volume of 4.0 L at 5.0 atm.
How to solve the problem of the gas law?
Gas Law Problems 1 (1) By rearranging the equation we can get, P=nRT/V 2 (2) Write down all the values which are known in S.I unit n= 1 R= 8.314J/K/mol T= 20degree celcius= (20+273.15)K=293.15K V=1L=0.001m 3 3 (3) Put all the values in the equation
Which is the formula for the ideal gas law?
Basically, the ideal gas law gives the relationship between these above four different variables. V = volume of gas. T = temperature of the gas. P = pressure of the gas. R = universal gas constant. n denotes the number of moles. We can also use an equivalent equation given below. Where, k = Boltzman constant and N = number of gas molecules.
Last Update: Oct 2021
Leave a reply
Comments
Shekera
24.10.2021 00:29Equally the name states the law is applicable under the ideal conditions, non to real gases.