Are you searching for 'more stoichiometry homework number 2 answers'? You can find your answers here.
Table of contents
- More stoichiometry homework number 2 answers in 2021
- Stoichiometry worksheet pdf
- Stoichiometry worksheet 2 answers
- Mole-mole stoichiometry worksheet answers
- Stoichiometry worksheet answers
- Solution stoichiometry worksheet with answers pdf
- Ws stoichiometry #2 answer key
- Stoichiometry worksheet with answers pdf
More stoichiometry homework number 2 answers in 2021
 This picture illustrates more stoichiometry homework number 2 answers.
This picture illustrates more stoichiometry homework number 2 answers.
Stoichiometry worksheet pdf
 This image illustrates Stoichiometry worksheet pdf.
This image illustrates Stoichiometry worksheet pdf.
Stoichiometry worksheet 2 answers
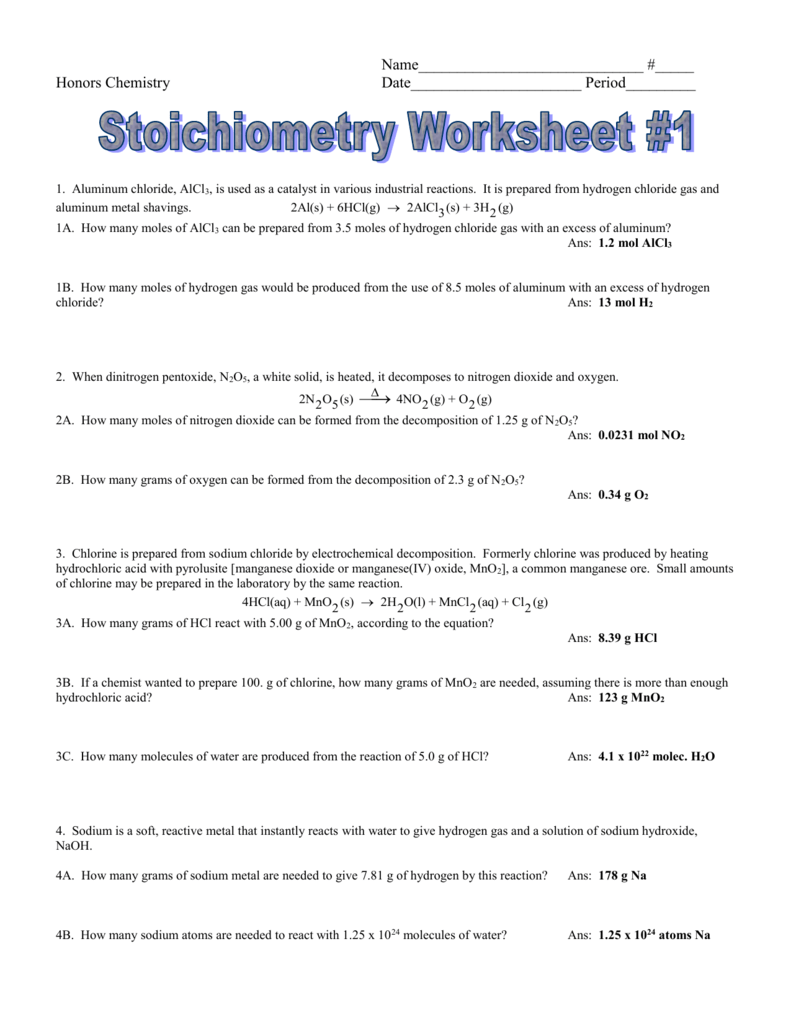 This image illustrates Stoichiometry worksheet 2 answers.
This image illustrates Stoichiometry worksheet 2 answers.
Mole-mole stoichiometry worksheet answers
 This picture shows Mole-mole stoichiometry worksheet answers.
This picture shows Mole-mole stoichiometry worksheet answers.
Stoichiometry worksheet answers
 This image representes Stoichiometry worksheet answers.
This image representes Stoichiometry worksheet answers.
Solution stoichiometry worksheet with answers pdf
 This image shows Solution stoichiometry worksheet with answers pdf.
This image shows Solution stoichiometry worksheet with answers pdf.
Ws stoichiometry #2 answer key
 This picture shows Ws stoichiometry #2 answer key.
This picture shows Ws stoichiometry #2 answer key.
Stoichiometry worksheet with answers pdf
 This picture demonstrates Stoichiometry worksheet with answers pdf.
This picture demonstrates Stoichiometry worksheet with answers pdf.
How to calculate the stoichiometry of 2 methoxy-2-methylpropane?
A 4.43 g sample of 2-methoxy-2-methylpropane (formula C_5H_ {12}O), is mixed with 119.19 atm of O_2 in a 1.64 times 10^ {-1} L combustion chamber at 327.9 degrees C. The combustion reaction to CO_2 a... What volume of O_2 at 836 mm Hg and 41^oC is required to synthesize 13.5 mol of NO? Express your answer numerically in liters.
Why are masses converted to moles in stoichiometry?
When performing a mass to mass stoichiometry problem, why masses must be converted to moles rather than directly using the mass of reactants to predict the mass of products. Consider the synthesis...
How to test your understanding of stoichiometry questions?
Test your understanding with practice problems and step-by-step solutions. Browse through all study tools. What is the molarity of sodium ions in 4.57 L of a 0.847 M Na3P solution?
Which is the correct equation for stoichiometry equation?
1. Given the following equation: 2 C4H10 + 13 O2 ( 8 CO2 + 10 H2O Write the following molar ratios: a. C4H10 ____mol C4H10 O2 mol O2 b. O2 ____mol O2
Last Update: Oct 2021