Are you searching for 'synthesis of salicylic acid from benzene'? You will find all the information on this section.
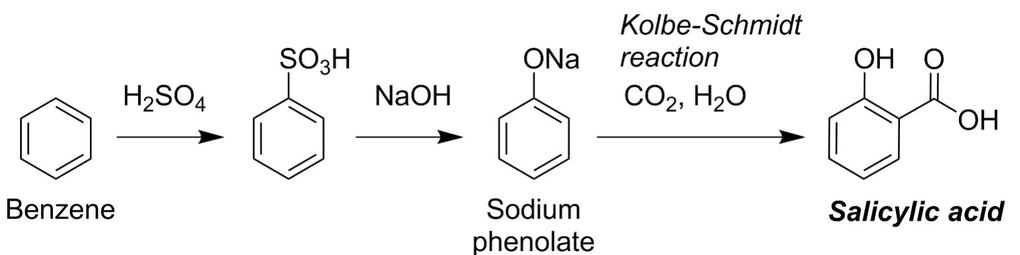
1st, a reaction betwixt BenzeneBenzene is Associate in Nursing organic chemical odd-pinnate with the natural science formula C₆H₆. The benzene molecule is composed of half-dozen carbon atoms coupled in a band with one H atom attached to each. As information technology contains only atomic number 6 and hydrogen atoms, benzene is classed as a hydrocarbon. and sulphuric acrid is carried exterior to obtain benzine sulphonic acid. Past benzene sulphonic vitriolic is reacted with sodium hydroxide fashionable which sodium phenolate is obtained every bit the product. Ulterior sodium phenolate is reacted with atomic number 6 dioxide and body of water in which the product obtained is salicylic acid.
Table of contents
- Synthesis of salicylic acid from benzene in 2021
- Synthesis of methyl salicylate lab
- Synthesis of aspirin mechanism
- Phenol to salicylic acid mechanism
- Salicylic acid to aspirin
- Synthesis of ibuprofen from benzene
- Uses for salicylic acid
- Acetylsalicylic acid to salicylic acid
Synthesis of salicylic acid from benzene in 2021
 This image demonstrates synthesis of salicylic acid from benzene.
This image demonstrates synthesis of salicylic acid from benzene.
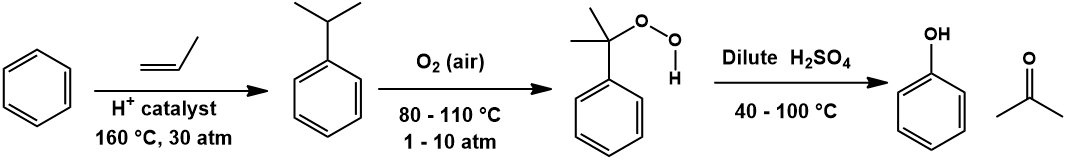
Synthesis of methyl salicylate lab
 This image illustrates Synthesis of methyl salicylate lab.
This image illustrates Synthesis of methyl salicylate lab.
Synthesis of aspirin mechanism
 This picture representes Synthesis of aspirin mechanism.
This picture representes Synthesis of aspirin mechanism.
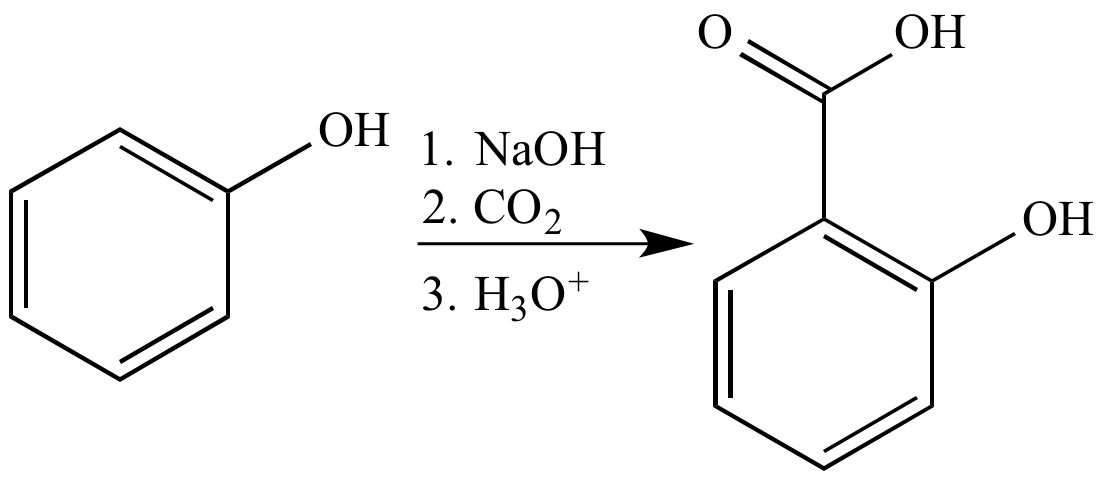
Phenol to salicylic acid mechanism
 This picture representes Phenol to salicylic acid mechanism.
This picture representes Phenol to salicylic acid mechanism.
Salicylic acid to aspirin
 This picture demonstrates Salicylic acid to aspirin.
This picture demonstrates Salicylic acid to aspirin.
Synthesis of ibuprofen from benzene
 This image demonstrates Synthesis of ibuprofen from benzene.
This image demonstrates Synthesis of ibuprofen from benzene.
Uses for salicylic acid
 This image demonstrates Uses for salicylic acid.
This image demonstrates Uses for salicylic acid.
Acetylsalicylic acid to salicylic acid
 This image representes Acetylsalicylic acid to salicylic acid.
This image representes Acetylsalicylic acid to salicylic acid.
How can we synthesize salicylic acid from benzene?
How can we synthesize salicylic acid from benzene? Salicylic acid is an important starting material in the synthesis of Aspirin. The key step in the laboratory synthesis of salicylic acid is the Kolbe-Schmitt reaction between phenol and a carbon dioxide.
Which is formed when benzene reacts with sulphuric acid?
When Benzene (C6H6) reacts with Sulphuric Acid (H2SO4) , we get BenzeneSulphonic Acid (C6H5SO3H). The obtained BenzeneSulphonic Acid (C6H5SO3H) on further reaction with Sodium Hydroxide (NaOH), Sodium Phenolate (C6H5ONa) is formed.
How do you get salicylic acid from baking soda?
But if you just need salicylic acid, buy some aspirin, boil it with a stoichiometric amount of baking soda for ten minutes, and then neutralize the resulting solution with HCl. The salicylic acid will precipitate as the solution cools.
How do you get phenol from salicylic acid?
In the formula of these two compounds the extra thing that we are seeing is CO2 in salicylic acid. By removing CO2 from salicylic acid we will get phenol. The process of removing CO2 is called decarboxylation which is done with the reagent NaOH + CaO. Feel free to comment if needed. (; How do you write a conversion of benzene to salicylic acid?
Last Update: Oct 2021